Chlorine Form At Room Temperature
Chlorine chlorine physical and chemical properties.
Chlorine form at room temperature. Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature. The second lightest of the halogens it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. It has a choking smell and inhalation causes suffocation constriction of the chest tightness in the throat and after severe exposure edema filling with fluid. Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
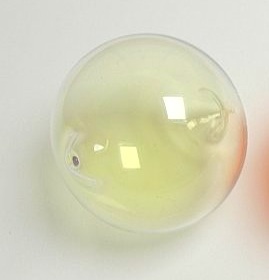
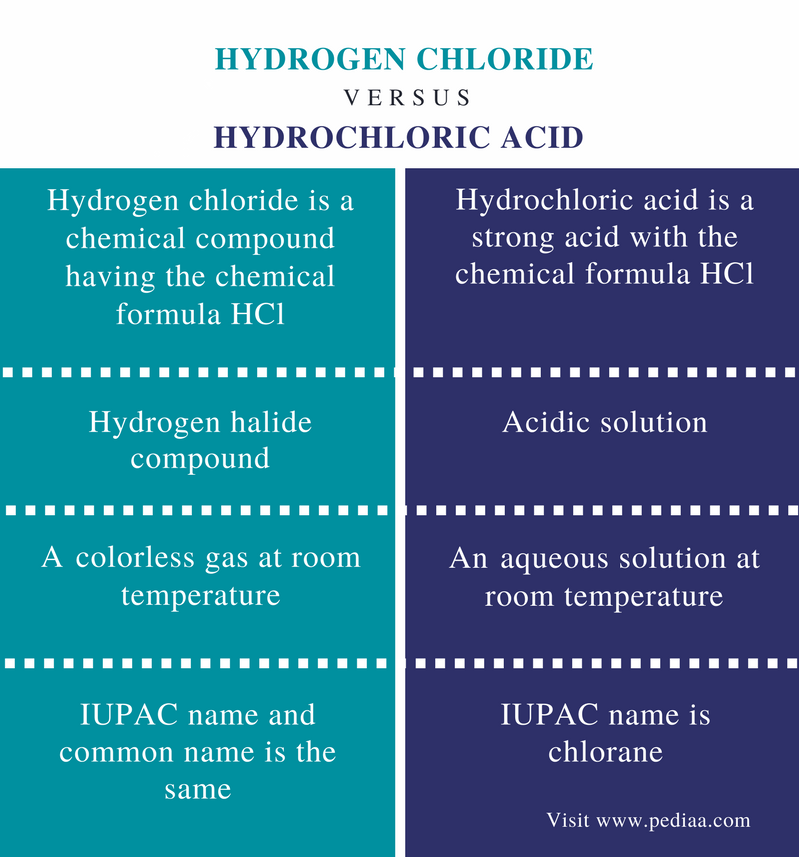
Chlorine was first produced by carl wilhelm scheele a swedish chemist when he combined the mineral pyrolusite mno 2 with hydrochloric acid hcl in 1774 although scheele thought the gas produced in his experiment contained oxygen sir humphry davy proved in 1810 that it was actually a distinct. Since it combines directly with nearly every element chlorine is never found free in nature. At room temperature chlorine is a yellow green gas that is heavier than air and has a strong irritating odor. Chlorine is a yellow green gas at room temperature.
Chlorine is a yellow green gas at room temperature. Relative atomic mass the mass of an atom relative to that of. The density of chlorine gas is approximately 2 5 times greater than air which will cause it to initially remain near the ground in areas with little air movement. It is two and a half times heavier than air.
The temperature at which the liquid gas phase change occurs. It becomes a liquid at 34 c 29 f. Chlorine is a naturally occurring element found in gaseous form at room temperature. A strong oxidizing agent chlorine deactivates microorganisms by breaking through the cell membrane.
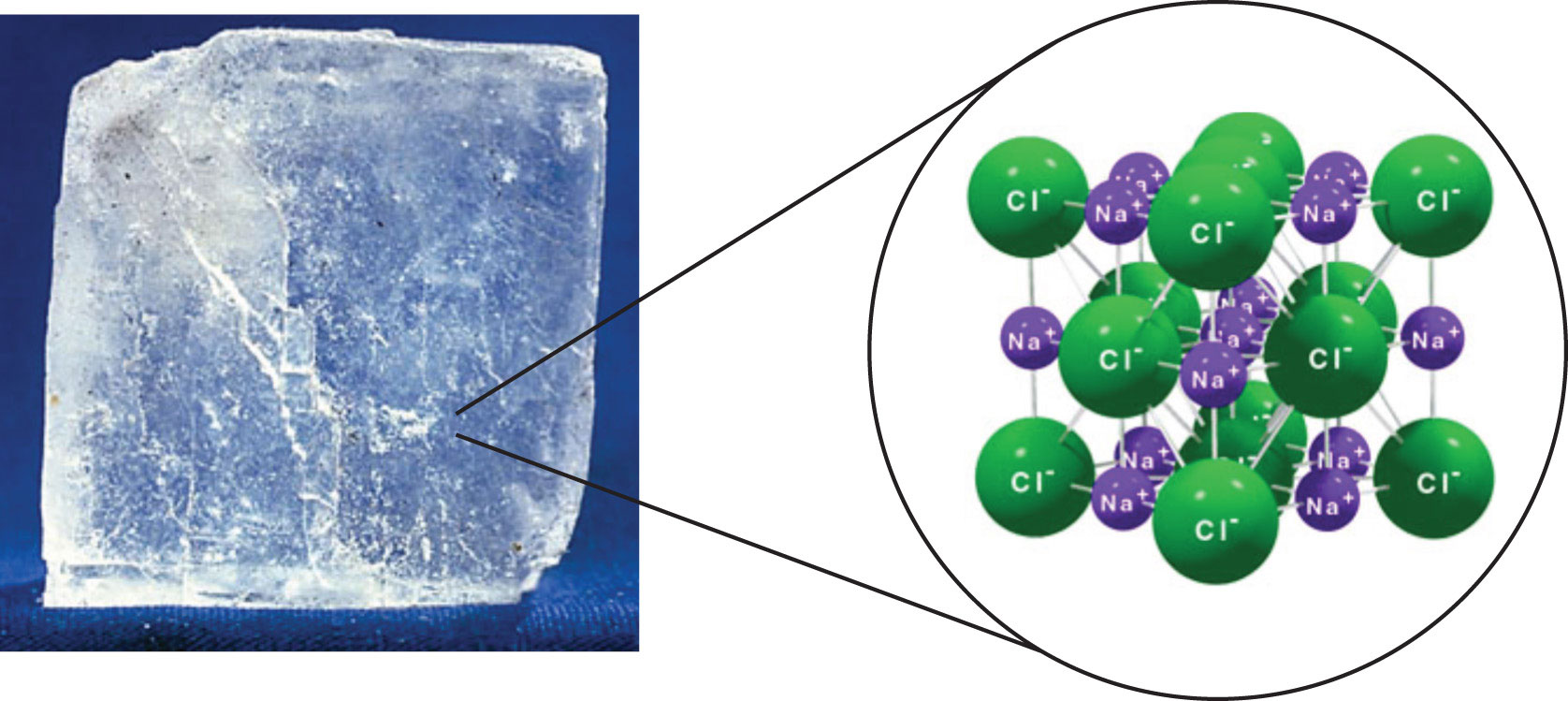
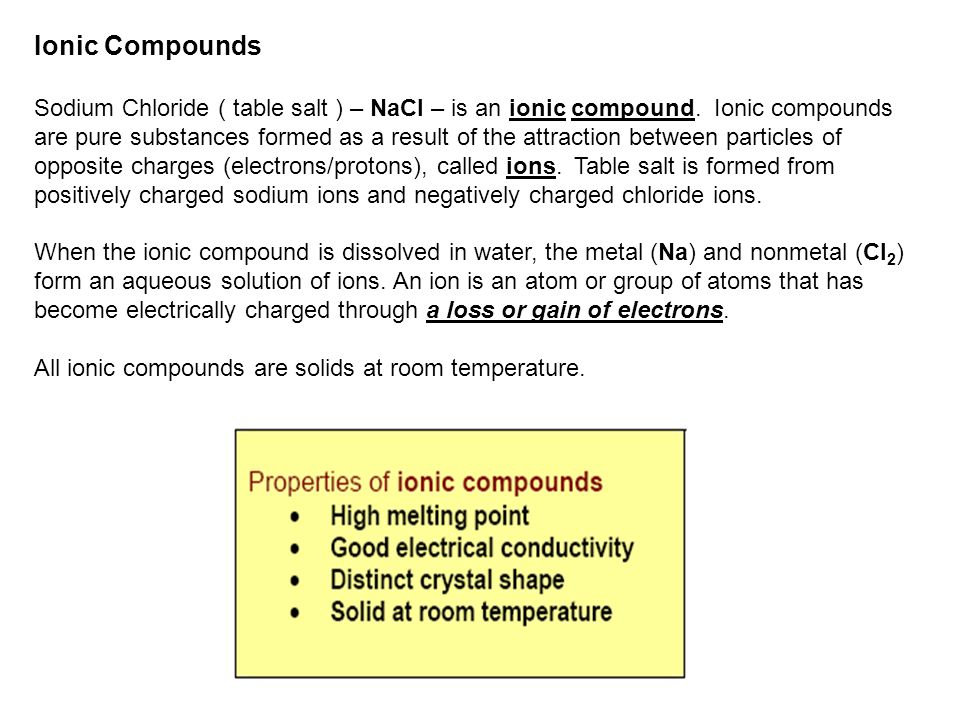
Chlorine has a pungent irritating odor similar to bleach that is detectable at low concentrations. Chlorine is mainly used as bleach in the manufacture of paper and cloth and to make a wide variety of products. The highly reactive nature of chlorine means it is usually bound with other elements such as sodium chloride salt. It is an extremely reactive element and a strong oxidising agent.
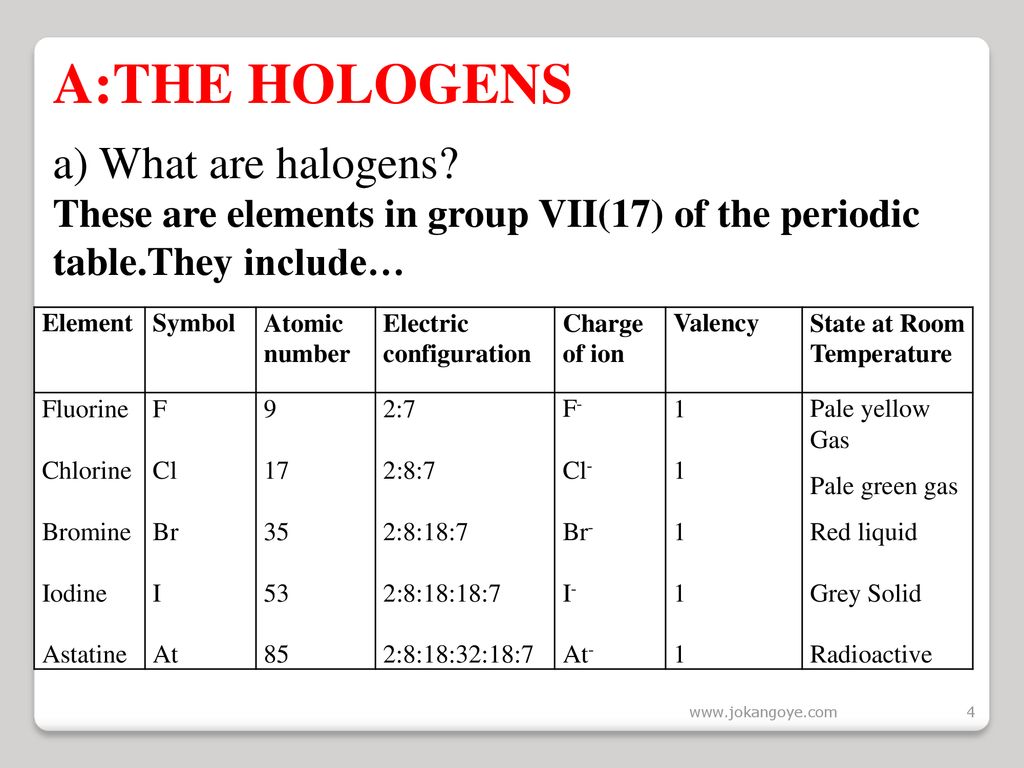
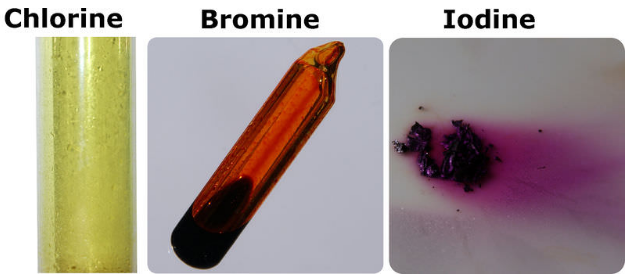
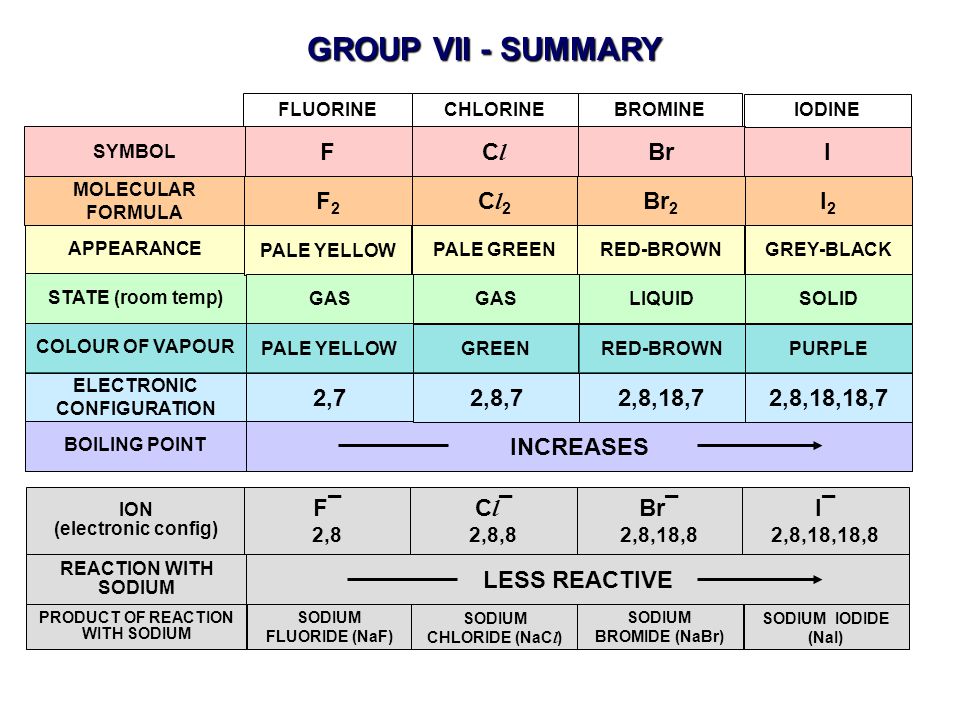
Among the elements it has the highest electron. 2 9 recall the colours and physical states of the elements at room temperature chlorine gas pale green bromine liquid red brown evaporates to form brown gas iodine solid black sublimes to form purple gas 2 10 make predictions about the properties of other halogens in this group reactivity decreases down the group color darkens down the group.